From the early months of the COVID-19 pandemic, the transmissibility and severity of SARS-CoV-2 variants in vulnerable populations have been the topic of extensive publications. Paediatric infection initially received substantial attention in the literature, as children are known to experience influenza-like illness (ILI) and severe acute respiratory infections (SARI) from many other pathogens (1, 2), with pneumonia being the leading infectious cause of death in children aged <5 years (3). Since that time, multicentre studies (4), prospective cohort studies (5) and systematic reviews (6-8) have demonstrated that SARS-CoV-2 variants circulating in 2020 generally had a mild clinical course in children.
The Delta variant of SARS-CoV-2 was first identified in India in October 2020 (9). Shortly after its identification as a Variant of Concern (VOC) by the World Health Organization (WHO) in May 2021 (9), the Delta variant trended towards global predominance, documented in 191 countries by October of the same year (10). A global analysis estimated a 55% increase in Delta’s effective reproduction number when compared to the previously dominant Alpha variant (11), and a pre-print study of north-eastern United States of America (USA) data estimated Delta to be 58-120% more transmissible than Alpha, depending on population characteristics (12). The Delta variant has a more severe clinical course at a population level. A national cohort study in the United Kingdom (UK) found that hospital admission within 14 days of COVID-19 diagnosis was two times more likely in confirmed Delta cases compared with confirmed Alpha cases (13). A study of national surveillance data in Singapore found increased disease severity, a composite outcome combining morbidity (requiring supplemental oxygen or intensive care unit (ICU) admission) and mortality, in Delta compared to wild type strains (14). Furthermore, a retrospective analysis of national surveillance data in Canada demonstrated that the Delta variant was associated with increased risk of ICU admission and death when compared to previous variants (15).
The literature specific to childhood infection with Delta is not as extensive as that relating to the population overall (16). Despite this, public interest in the impact of COVID-19 on children remains high. Academics, clinicians, epidemiologists, the Australian Medical Association and the Australian Technical Advisory Group on Immunisation have been called upon (17, 18) to provide evidence-based advice on the impact of Delta on children. This advice, and its impact on vaccination, education, and masking policies, has varied widely across the globe. While a number of experts and peak bodies have urged vaccination of young people against COVID-19 (19-21), others have refrained. A quantitative risk-benefit analysis conducted in England in 2021 concluded that in the setting of high infection incidence ((1000/100,000 population/week over 16 weeks), vaccination could prevent 4,430 hospital admissions and 36 deaths over 16 weeks, and hence the benefits of vaccination substantially outweigh the risks (22). The United Nations Educational, Scientific and Cultural Organization (UNESCO) has reported that almost half of schools globally had implemented partial or total closures in response to the pandemic, with a disproportionate impact on the most vulnerable learners (23) Regional branches of the WHO and United Nations Children's Fund (UNICEF) in August 2021 urged schools in Europe and Central Asia to offer face-to-face learning, and instead bolster infection control policies to counter Delta’s increased transmissibility (24). The American Academy of Pediatrics (AAP) also advocated for a return to face-to-face education, citing evidence of low COVID-19 transmission rates in schools when appropriate prevention measures were implemented (25). Specific school-based mitigation measures of daily symptom screening, teacher masking and closure of extracurricular activities in the United States showed a significant reduction in risk of COVID-19-related outcomes (26). Mask mandates for children have been similarly diverse, with the AAP and the Centers for Disease Control and Prevention (CDC) in the USA recommending universal masking for children aged >2 years in educational facilities (21, 27), and the WHO recommending children aged <5 years should generally not be required to mask, and children aged 5-11 years mask under certain conditions (28). Government mask mandates reflect this lack of consensus, commencing at age 6 years in South Africa (29) and Singapore (30), 12 years in Australian states (31), and as of July 2021, no longer mandated at any age in England outside of health facilities, although this changed temporarily during the Omicron variant wave in December 2021 (32).
To date, there has been no systematic review of the transmissibility and severity of infection with the Delta variant of SARS-CoV-2 in children. This review seeks to fill that gap, and in so doing contribute to the evidence base available to those determining policy and practice. Key outcomes include attack rate, hospitalisation rate and mortality rate in children worldwide. The implications of these findings may inform decision making on policies pertaining to paediatric mask mandates, school closures, and vaccination strategies.
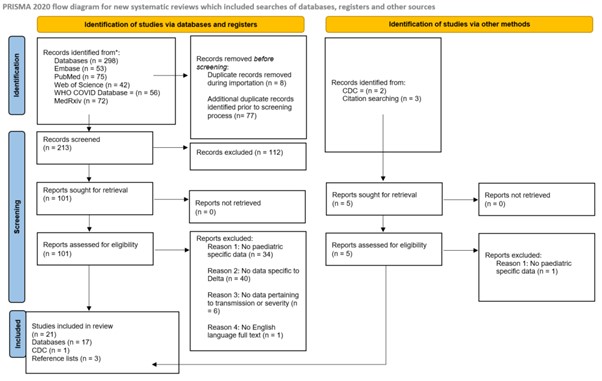
Databases including PubMed, Embase, medRxiv, Web of Science and WHO COVID database were searched for articles from the period October 2020 to March 2022 to identify all published articles that reported evidence of the SARS-CoV-2 Delta variant among the paediatric population. Additionally, reference lists of eligible studies and grey literature were hand searched for additional studies for inclusion. The study then followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) reporting guidelines (33) depicted in Figure 1. Search results were downloaded to EndNote version 20 (Clarivate, Philadelphia, United States). Studies were compared to pre-defined inclusion and exclusion criteria at each stage, and duplicate studies removed. Study selection was undertaken by three independent reviewers (DH1, EB & DH2), with disagreements resolved by consensus.
Table 1
Search terms based on PICO framework
| Population | (Adolescent OR infant OR child OR pediatric OR paediatric OR newborn OR preschool child OR school) |
| Intervention | (Infection OR disease) |
| Comparator | None specified |
| Outcome | (SARS-CoV-2 OR COVID-19 OR severe acute respiratory syndrome coronavirus 2 OR coronavirus disease 2019 OR Delta OR SARS-CoV-2 variants OR B.1.617.2) |
PRISMA flow diagram
Search terms were defined by three reviewers based upon our PICO framework (DH1, EB & DH2) who then conducted the search independently. Consensus was reached in selecting the studies for inclusion by discussion (Table 1).
Eligibility criteria included English language literature, inclusive of all countries of publication, and within publication dates of October 2020 (month that the first case of Delta was identified worldwide) to March 2022 (month that the review was concluded) (Table 2). To satisfy eligibility criteria, sources needed to present epidemiological data including but not limited to infection, transmission, or severity (including hospitalisation and death) with probable or confirmed cases of the Delta variant of SARS-CoV-2, in children (aged up to 9 years) or adolescents (aged 10 to 19 years). Studies presenting data that compared trends between Delta and other SARS-CoV-2 variants were included. Eligible study types included case series, case reports, case-control studies, and cross-sectional studies. Exclusion criteria included all mathematical modelling studies and literature published in a language other than English. Sources were ineligible if they contained no relevant data, duplicate studies or data, or inadequate demographic information to delineate data pertaining to adults versus children. A total of 21 studies were selected for inclusion.
Table 2
Eligibility criteria
| Inclusion | Exclusion |
| Articles published from October 2020 to March 2022 | All mathematical modelling studies |
| Original articles, case series, case reports, case-control studies, and cross-sectional studies | Literature published in a language other than English |
| Published in English | Duplicate studies |
| Full-text freely available online or through a library subscription. | Inadequate data |
| Articles must provide adequate detail of epidemiological data including but not limited to infection, transmission, or severity (including hospitalisation and death) with probable or confirmed cases of the Delta variant of SARS-CoV-2, in children (aged up to 9 years) or adolescents (aged 10 to 19 years). |
Data sought for extraction, depending on availability, included study citation; publication month and year; study type; country of origin; participant characteristics (age and sex); sample size; vaccination status; and outcomes, including incidence, secondary attack rate, hospitalisation, ICU, and mortality rates. Data were checked for accuracy by a second reviewer.
Risk of bias was determined using the Joanna Briggs Institute Critical Appraisal Guidelines for assessing bias (34). This tool was used due to the versatility of checklists available to critically appraise different study designs. Risk of bias was independently assessed by two authors (DH1 and DH2). A third reviewer (EB) was available for consultation if consensus was not initially reached.
Ethics approval was not applicable.
In all, 298 studies were identified from 5 databases and grey literature searches. A total of 85 studies were removed as duplicates; 213 articles were screened; 101 were sought for retrieval; 101 were then assessed for eligibility; a further 80 studies were then excluded, and 21 studies were included in the final review (Figure 1). Overall, the 21 included studies spanned 7 countries and the European Union with settings comprising school, households, childcare facilities and summer camps in this review (35-55). The studies included 449,198 paediatric cases of infection with the delta variant of SARS-CoV-2, with a reported mortality rate of 0.01% (35, 50), which increased to 1.1 to 1.8% of hospitalised patients (38, 55). A summary of included studies reporting on cases by age and sex is available in Table 3. A detailed summary of all included studies including their characteristics is available in Table 4 and data on outcome measures are summarised in Table 5, which are provided as Supplementary Material. A narrative synthesis approach was chosen to report on incidence, cases by age and sex, attack rate, markers of severity (hospitalisation, ICU admission and mortality), paediatric cases as a proportion of total cases, and transmission in households and educational facilities.
Table 3
Overview of Cases by age, sex and setting
| Authors | Country | Setting | Type of study | Summary points |
| National Centre for Immunisation Research and Surveillance (2021) (36) | Australia | Schools and educational facilities | State data report | In NSW children with COVID-19 in June and July 2021, older children accounted for the highest proportion, with 28% aged 0-5, 33% aged 6-12, and 39% aged 13-18. Conversely, in NSW educational facility outbreaks in the same period, early learning centres enrolling children aged 0-5 had the highest secondary attack rates amongst children (4.9%). Significantly lower transmission rates were observed in primary schools (1.6%), while no secondary transmission was recorded in secondary school settings (0%). Secondary students were required to wear masks. Schools were open for face-to-face teaching of vulnerable children and children of essential workers. |
| Dougherty et al. (2021) (41) | USA | Gymnasium | Retrospective cohort | A USA gymnasium outbreak noted higher case numbers in older children, with 64.5% (20/31) of cases of secondary and tertiary transmission in the 12-19 age group compared to 35.4% (11/31) in the 5-12 age range. |
| Li et al. (2021) (43) | China | Hospital | Retrospective cohort | A comparative analysis of 226 SARS-CoV-2 Delta infected people in Putian, China, of which 77 were under the age of 12 years reported males accounted for 57.1% of the cases with a median age of 9 years. |
| Loconsole et al. (2021) (45) | Italy | National data | Retrospective cohort | At a regional population level in Italy during the Delta wave, younger children accounted for a lower proportion of COVID-19 diagnoses, with 4.4% aged 0-4 years and 16.4% aged 5-16 years. |
| Cheng et al. (2022) (46) | China | Hospital | Retrospective cohort | A comparative analysis of 66 paediatric patients infected with SARS-CoV-2 Delta variant (30 boys and 36 girls with a mean age of 10.4 years) and 23 paediatric patients in 2020 with original strain SARS-CoV-2 (14 boys and 9 girls with a mean age of 6.2 years). |
| Tonzel & Sokol (2021) (47) | USA | Youth summer camps | Outbreak report | An outbreak in US youth summer camps recorded 321 camp-associated cases, where 85.4% of cases occurred among campers with a median age of 12-years during the SARS-CoV-2 Delta surge in Louisiana. |
| Essa et al. (2021) (42) | Iraq | Hospital | Case study | A case study from an infant in Iraq diagnosed with the Delta variant who had co-infections leading to death. |
| Kumar {Kumar, 2021 #47}et al. (2021) (48) | India | National data | Cross sectional | An analysis of SARS-CoV-2 Delta infections amongst an Indian population reported a lower incidence in children, with the 0-9-year age group accounting for 4% males and 5% females and the 10-19-year age group 9% males and 10% females out of 6,238 infections. |
| Glatman-Freedman et al. (2021) (49) | Israel | National data | Retrospective cohort | An Israeli study reported on a 12-15-year-old cohort of 8,144 SARS-CoV-2 infections during the Delta wave while vaccinating the adolescent population. High vaccine effectiveness against infection and disease severity observed in this population. |
| Olson et al. (2021) (50) | USA | Hospital | Retrospective cohort | A US study reporting on hospitalisation rates among persons aged 12-18 years who were vaccinated during the Delta surge reported 19 case-patients with median age of 15 years. |
| Molteni et al. (2021) (51) | UK | National data | Retrospective cohort | A prospective COVID-19 symptom study in the UK reported 227 children aged 5-11 years and 479 children aged 12-17 years who had confirmed SARS-CoV-2 Delta infections throughout May to July 2021 nothing a higher incidence in the older adolescent group. |
| Naleway et al. (2021) (52) | USA | Hospital | Retrospective cohort | An analysis of SARS-CoV-2 infections by age group amongst the Oregon and Washington population reported 472 infections amongst the 12-17-year age group between July-September, 2021 out of 7,155 laboratory-confirmed infections. |
| Edward et al. (2021) (53) | USA | Hospital | Retrospective cohort | A single site analysis in a Chicago children's hospital found 2,025 patients positive with SARS-CoV-2 where 46.5% were confirmed to have the Delta variant. |
| Hao et al. (2022) (54) | China | Hospital | Retrospective cohort | A comparative analysis in China reported on 66 paediatric patients hospitalised with confirmed SARS-CoV-2 Delta infection during July-August 2021. |
| Bundle et al. (2021) (55) | European Union | National data | Retrospective cohort | A retrospective cohort analysis from the European Union of 820,404 symptomatic SARS-CoV-2 infections in children aged 0-17 years, reported the highest incidence in the 12-17-year-old cohort with 419,568 symptomatic cases. |
Twenty-one studies were assessed overall with all studies meeting the requirements for critical appraisal. Reviewers (DH1 & DH2) followed the Joanna Briggs Institute Critical Appraisal Guidelines to determine whether each question outlined in the respective checklist for the study design received a “yes”, “no”, or “unclear” answer. Studies were considered to be of high quality if 80-100% of responses to the critical appraisal questions were “yes”, and of moderate quality if 50-79% of the responses were “yes”. Only studies that were appraised as high or moderate quality were included in this review. This review appraised cohort and cross-sectional studies and case reports. Cohort studies were required to have at least six “yes” answers to be included in this review. Cross-sectional studies and case reports were included if four or more questions were answered as “yes”. The methodological quality rating by study design used to assess the 21 studies is provided in Table 6 of the Supplementary Material. Overall, all 12 cohort studies we assessed as ‘moderate’. For cross-sectional studies, 7 were assessed as ‘moderate’ and 2 were assessed as ‘high’. A risk of bias summary for all studies is provided in Table 7 of the Supplementary Material.
Two studies provided information on incidence at national (USA) or state (NSW) levels and two studies provided incidence amongst populations experiencing outbreaks. Close to half a million paediatric COVID-19 cases were recorded in the USA in the Delta wave predominant two-week reporting period during September 2021, an 8% increase (from 5,292,837 to 5,725,680) in cumulative cases since reporting began in April 2020 (35). The proportion of paediatric cases compared to total USA cases increased over time from 2.6% in April 2020 to 26.7% in September 2021 (35). The proportion of the population aged under 18 was 22.1% in 2020 (56). In the Australian state of New South Wales (NSW), the proportion of COVID-19 cases occurring in those aged <16 years during the Delta predominant study period (June – August 2021) was similar, at 26.6% (36). The number of summer camp-associated cases in Louisiana in June-July 2021 was 31 times greater than the same period in 2020 (47). Amongst 12-17-year-olds in Oregon and Washington, the incidence of SARS-CoV-2 infection (per 1000 people) in July-September 2021 was 3.2 (95% CI: 2.4-4.2) in vaccinated persons and 27.9 (95% CI: 25.4-30.7) in unvaccinated persons (52).
Data on age was available from fifteen studies, while sex data was provided in three studies as summarised in Table 3. The included studies spanned educational facilities (36), a U.S. gymnasium (41) and outbreaks across US youth summer camps (47). One study reported on a single SARS-CoV-2 Delta variant case in an infant in Iraq (42). Seven studies reported population-level statistics of Delta variant infections in the paediatric population of each country respectively (45, 48-50, 52-54). One study reported on Delta variant infections in Putian, China, noting that the virus spread from children in schools to workers in factories via family contact (43). A further study from China compared SARS-CoV-2 Delta variant infections to original strain infections (46). One study reported data from the UK national COVID symptom study (51). A final study analysed Delta variant infections across the European Union (55).
Six studies provided data from which attack rates could be derived. A joint report from 49 US states reported publicly available data on children with COVID-19 diagnoses. State testing numbers showed that while children represented 16% of cumulative COVID-19 cases, the overall attack rate remained low at 0.58% (35). Overall, attack rates amongst children in NSW educational facility outbreaks were highest when the primary case was an adult, at 7.0%. The attack rate was higher still at 8.1% when looking specifically at outbreaks in early learning centres with an adult primary case. Comparatively, the attack rate was just 1.6% amongst children in outbreaks where the primary case was also a child, increasing to only 1.8% in early learning settings (36). In a US study of an elementary school outbreak, the attack rate was 54.5% in the class where the index case, a teacher, was located. Transmission occurred through unknown means to a secondary class, in which the attack rate amongst tested children was 42.8% (40). A gymnasium-based outbreak recorded a secondary attack rate of 18.8% amongst gymnasts (41). Reporting from emergency department visits in Oregon and Washington between July and September 2021 saw an attack rate of 0.3% of the 15,234 presentations who were vaccinated compared to 2.8% of the 15,179 presentations who were unvaccinated between ages 12-17 years (52).
Data pertaining to hospitalisation, ICU admission or mortality was available from twelve of the 21 studies. One study of national USA hospitalisation and mortality rates provided data as a cumulative percentage of COVID-19 cases by week. The rate of cumulative paediatric hospitalisation decreased throughout 2020, then remained steady at 0.8% from January 2021, later increasing to 0.9% in June 2021. However, this rate was not differentiated for children. This increase occurred prior to Delta becoming predominant in the United States and remained stable thereafter. Cumulative child mortality rates were 0.01% prior to Delta’s detection in the US and remained at 0.01% after Delta’s assumed predominance (35). Age ranges reported for children varied by state (0-14, 0-17, 0-18, 0-19, and 0-20 years), therefore more detailed data of hospitalisation rates was unavailable (35).
An analysis of hospitalisation rates per 100,000 children across 14 US states noted weekly hospitalisation rates were lowest in June/July 2021 at 0.3 hospitalisations per 100,000 children and peaked in August 2021 at 1.4 hospitalisations per 100,000 children as Delta assumed predominance (38). This increase was most marked in the 0-4-year age group, where the hospitalisation rate per 100,000 children increased from 0.2 to 1.9 between June and August (38). Adolescents hospitalised during the June-July period were more likely to be unvaccinated 86.8% (59/68), compared with partially 7.4% (5/68) or fully vaccinated 5.9% (4/68) (38). A separate study of trends in US hospital presentation and admission in the year to August 2021 found that vaccination coverage of those aged >12 years was associated with lower COVID-19 associated emergency department (ED) visits and hospital admission rates (39). This study also reported a bimodal distribution of hospitalisation rates in the paediatric population, with the majority of admissions in those aged <4 years and 12-15 years (39).
The impact of vaccination on hospitalisation rates was measured in three studies. A large study in Israel demonstrated a higher proportion of unvaccinated (33/9969) cases were hospitalised, compared to vaccinated adolescents (0/1117) (47). A US study in Oregon and Washington reported the incidence rate ratio (IRR) for adolescents aged 12–17 years between July 4 to September 25, 2021, to be 8.87 (95% CI: 6.58–11.94), which was more than double that compared with other strata including sex and race (50). COVID-19 hospitalizations were rare among fully vaccinated adolescents and young adults, while hospitalisation was more evenly distributed across age ranges in the unvaccinated group (50). Total healthcare encounters were less for the vaccinated group of adolescents (50).
Prior to the Delta period in NSW, Australia SARS-CoV-2 transmission remained low across educational settings without any hospitalisations recorded. At the commencement of the Delta period on June 16, 2021, up to August 19, 2021, 2,864 COVID-19 cases were diagnosed in children, representing 27% of all COVID-19 cases diagnosed in NSW (36). Of these COVID-19 cases, 810 (28%) were aged 0–5 years, 945 (33%) were aged 6–12 years and 1,109 (39%) were aged 13–18 years (36). Of these children diagnosed with COVID-19, 70 were admitted to hospital. Two of these were newborns born in hospital, 68 were from the community and 25 were admitted for social reasons, thus, the effective hospitalisation rate of children admitted from the community for medical reasons was 2.4% (70/2864) (36). Five children across NSW required ICU admission, all of whom were aged 15-18 years and were unvaccinated, and some of whom had underlying medical conditions (36). A family cluster analysis found that one 15-year-old child (1/6) required hospitalisation, who was noted to have comorbidities including overweight and epilepsy. He did not require ICU admission and was discharged on day 3 of hospitalisation (37). In the case study of a 6-year-old hospitalised child, discharge occurred after 7 days, also without requiring ICU admission (42). No children from the gymnasium (41) or primary school (40) outbreaks required hospitalisation. In a retrospective single centre cohort study conducted in a children's hospital in Chicago, USA, 2,025 COVID-19 positive patients were identified from October 2020 to August 2021. Of these, 409 samples were sequenced, with 119 Delta infections recorded. Only 8 of these experienced moderate or severe disease (many were hospitalised for conditions other than COVID-19), and Delta was not shown to result in an increase in severe disease compared to other variants (51).
A retrospective analysis of pooled case-based surveillance data reported to the European Surveillance System, that included more than 800,000 paediatric cases between August 2020 and October 2021, showed that while hospitalisations rose in 0–17-year-olds during the period of Delta predominance, it did so in proportion with increase in transmission, consistent with the full study period (55). Severe outcomes remained rare in the Delta period. There was an increased risk of hospitalisation in children under 1-year old (14.7% Delta vs 13.1% full period; p < 0.01) during this period, but not in other age groups. This study also compared children with comorbidities, and while there was an increased risk of hospitalisation for children with conditions such as diabetes and obesity, most children that were hospitalised did not have comorbidities.
In a prospective symptom study in the United Kingdom, comparison of symptoms was made between periods of Alpha and Delta variant predominance. While symptom burden was slightly higher with Delta than Alpha infection, there remained a low incidence of hospital presentation with either variant (16/706 for Delta) (51). A nationwide retrospective cohort study was conducted in Israel during the Delta outbreak. There were 11,086 laboratory confirmed COVID-19 cases aged 12-15 years during the four weeks to 26 August 2021. 33 cases were hospitalised, all were unvaccinated. No children died (49).
Two included studies were single case studies of hospitalised children, Fraser et al. described a patient with unusual progress of disease, and Essa et al. documented the first Delta case in Iraq (42, 44). From June-September 2021, with increasing numbers of U.S. paediatric COVID-19 hospitalizations, a test-negative-case-control study was conducted at 19 paediatric hospitals in 16 US states (50). Among the 179 patients aged 12–18 years hospitalised with COVID-19, six (3%) were vaccinated and 173 (97%) were unvaccinated, and in this group 29 became critically ill and two died, all unvaccinated (50).
Prior to March 2022, government health policy in China was to admit all confirmed cases of COVID-19 to designated hospitals (57), therefore hospitalisation is not an accurate proxy measurement for severity in studies of the paediatric population in China (43, 46, 54). When reported, ICU and mortality rates were low compared to adult rates, 0/66 (54) and 0/77 (43).
This study has reviewed the emerging literature on the transmissibility and severity of paediatric infections of the SARS-CoV-2 Delta variant. Studies reporting incidence data demonstrated at a national level in the USA, paediatric COVID-19 cases rose from 2.6% to 26.7% cumulatively leading to the end of September 2021 (35). Similarly at a state level in Australia, NSW COVID-19 cases peaked at 26.6% cumulatively by August end 2021 (36). Such a significant rise in COVID-19 incidence at both national (USA) and state (NSW) levels is alarming and demonstrates a strong need to collect incidence data for the paediatric population on all levels to determine how COVID-19 truly affects this population. It should be noted that these incidence rates may not explain the extent of the pandemic, as testing likely did occur for many asymptomatic children or those with mild symptoms. Studies reporting on attack rates within this review were limited due to the lack of available data at the time of review. Studies reporting on severity highlighted from twelve studies, rates of paediatric hospitalisation, ICU admission and mortality remain low following a Delta diagnosis. Interestingly the rate of paediatric hospitalisations prior to Delta being detected was 0.8% and rose to 0.9% in the USA, highlighting infection with the Delta strain does appear more likely to result in hospitalisation (35).
Overall, three key conclusions can be drawn from these results. The first is that paediatric COVID-19 diagnoses have risen as a proportion of total cases as Delta has assumed predominance, compared to previous SARS-CoV-2 variants. The second is that rates of paediatric hospitalisation, ICU admission and mortality remain low following a Delta diagnosis, although infection with the Delta variant does appear more likely to result in hospitalisation (35). The third is that Delta’s increased transmissibility has implications for paediatric exposure in household and educational settings. In addition, vaccination of both adults and adolescents has been shown to reduce paediatric hospitalisation rates at a population level (36). Whilst it is reported most COVID-19 cases in children are less severe and do not require hospitalisation there are still subgroups of this population that do experience significant illness and long-term consequences of COVID-19 infection.
The rise of Delta has coincided with an increasing proportion of COVID-19 cases being diagnosed in children. UNICEF’s COVID-19 database, comprising data from 106 countries, estimates that children and young people under 20 represent 20.6% of female COVID-19 cases and 22.4% of male COVID-19 cases diagnosed since the beginning of the pandemic (58). Children are thus underrepresented as a proportion of global cumulative COVID-19 cases, as children and young people comprise 33% of the global population in the same dataset (58). However, it is likely that cases are under-detected in children due to under-testing. Detecting paediatric cases may be particularly difficult due to the higher prevalence of asymptomatic infections in children, thus creating barriers to testing (59). However, once children were tested when schools began to open, they began to make up a proportion of cases that was consistent with their share of the population.
Studies in this review that analysed incidence during periods of Delta predominance noted children bore around a quarter of the Delta case burden in both Australia (26.6%) (36) and the USA (26.7%) (35). The growing proportion of COVID-19 cases being diagnosed in children may have been reflected by the impact of increasing adult vaccination rates and shift in epidemiology to children, although this does not solely account for the Australian study data (36). The total NSW vaccination rate was low at the beginning of the study period (5% single and 20% double vaccination of those aged >16 years), reaching moderate levels by the end of July (38% single and 18% double vaccination of those aged >16 years) (36).
Overall, children continue to experience low rates of hospitalisation and ICU admission following a COVID-19 diagnosis. In the USA, the cumulative percentage of hospitalisations in children compared to total US population has slowly risen over time, although it is important to note that this trend has been ongoing since reporting begun. Rates begun at a low of 0.8% in May 2020, rising to 1.9% when Delta was first identified in the USA in March 2021, and reaching a peak of 2.5% in September 2021 (35). Cumulative paediatric mortality in the USA has remained stable during Delta’s circulating period (35). It is worth noting, however, that 32.7% of recorded paediatric deaths due to COVID-19 occurred in the 12 weeks between the first week of July (when Delta became predominant) and the study period in late September, reflecting the increased volume of paediatric COVID-19 cases (35).
In NSW, medically indicated hospitalisation (2.4%) and ICU admission (0.17%) rates in children remained low during a prolonged Delta outbreak, however the data would likely not capture multisystem inflammatory syndrome seen in children (MIS-C) due to a delay in reporting of often up to 6-8 weeks (36). During the Delta outbreak, hospitalisations rose slightly, however this has been in part attributed to social reasons following parental hospitalisation (60), while ICU admissions remained at less than 1% across these ages in both time periods (61).
Severity data collected from sources (35) and (38-42) pertained to case studies or outbreaks with small numbers of cases (range: 1-23 cases), insufficient to draw conclusions about hospitalisation, ICU and mortality rates. Severity reporting from source (55) on 820,404 symptomatic COVID-19 cases in the European Union found only 1.2% were hospitalised, half of the 2.4% effective hospitalisation rate reported in NSW (36). The large population study in Europe showed that while hospitalisation rose during the Delta outbreak, apart from an increased risk of hospitalisation in the under 1 year age group, it was proportional to increasing community transmission (55). Most children hospitalised in this study did not report comorbidities, suggesting that high levels of community transmission may put otherwise healthy children at risk. However, increased number of hospital admissions may reflect increased transmissibility, rather than increased severity of Delta variant infection (53).
Vaccination, both of the adult population and of adolescents, appears to further reduce the incidence of paediatric hospitalisation (62). In the USA, increasing whole of population vaccination levels was associated with decreasing hospitalisation of children due to COVID-19, despite most children being vaccine ineligible by age. States with the lowest quartile of population vaccination coverage recorded 3.7 times the child hospitalisation due to COVID-19, compared to states with the highest quartile of population vaccination coverage (39). Vaccinated adolescents were also themselves less likely to be hospitalised due to COVID-19 (38), although these datasets compared hospitalisation trends over time and do not delineate Delta cases from other lineages. In three studies which looked at hospitalisation rates associated with vaccination status, there was less risk of hospitalisation in the vaccinated group, and less health care encounters in fully vaccinated adolescents (47, 48, 50). This demonstrates that vaccination is protective against disease severity for adolescents.
Several studies point to educational and household settings as key sites for paediatric infection with the Delta variant. One outbreak within an educational institution in Marin County, California reported where the primary case was a teacher the attack rate amongst children tested in the primary class was 54.4%, with 80% of those in the front row of their classroom reportedly positive and 28.6% of children seated in the third row of the classroom infected (40). However, one classroom adjacent to where the original outbreak occurred experienced an outbreak with an unidentified source where the attack rate was 42.3% (40). Of the unvaccinated children, no hospital or ICU admissions were recorded in the outbreak.
On a population level, 88.0% of NSW COVID-19 paediatric cases with a known source were acquired through a household contact (36). In NSW educational facility outbreaks where a child was the primary case (57.6%), the child’s source of infection was also primarily household transmission (70.6%) (36). Evidence of high levels of paediatric household transmission accords with a recent national UK-based all ages case control study, that found household transmission in clusters with a confirmed Delta index case compared to Alpha index case had an adjusted OR of 1.7 (95% CI 1.48-1.95, p <0.001) (63).
Transmission rates amongst NSW children in educational facilities and households during the 2021 Delta outbreak were noted to be 5.2 times higher than seen in 2020 (secondary attack rate 4.7% v 0.9%) (36). This aligns with all ages evidence on the Delta variant’s increased transmissibility, compared with Alpha (11, 12). The proportion of educational outbreaks occurring in early learning (62.8%) compared to primary and secondary educational facilities may reflect the impact of the lockdown of the capital city of Sydney beginning June 25th, nine days into the study period. Primary and secondary schools experienced holiday closures during the first two weeks of lockdown and opened only to vulnerable children and children of essential workers throughout the remainder of the study period. Conversely, early learning facilities remained open to all children throughout the lockdown period (36).
This review has several limitations. A wide range of study types were eligible for inclusion in this review, in order to cast a wide net and include as many studies as possible. As a result, outcome data was not directly comparable across studies. Additionally, included age ranges differed across studies, and sex data was not available for all studies for a direct comparison. Several included studies were based on US data, which may reduce generalisability of incidence and attack rate data in nations with different mask policies, school closures and vaccination rates. Limitations of studies utilising national USA data include a wide variation in age parameters, reported measures and reporting frequency between states, the inclusion of both confirmed and probable COVID-19 cases, and the reduction in frequency and completeness of data reporting from some states beginning in June 2021, just weeks before Delta became the dominant circulating variant in July 2021 (35). Due to insufficient data pertaining to outbreaks in educational and recreational settings at the time of literature searching overall attack rates could not be compared. Further, similar studies with meta-analyses to compare paediatric attack rates were unavailable to analyse paediatric cases as a proportion of total cases. Additionally, studies presenting national trends are by nature unable to provide a breakdown of outcome data by variant.
Future research could focus on decreasing the volume of paediatric COVID-19 cases by identifying the most efficacious risk reduction measures for children by age group. This may include delineating the ideal lower age limits for mask mandates, quantifying the impact of paediatric vaccination programs, and evaluating risk reduction strategies in household and educational settings.
Our review shows a growing proportion of COVID-19 cases were diagnosed in children in the context of the increased transmissibility of the SARS-CoV-2 Delta variant. Despite lower vaccination rates in this population during the study period compared to adults, children experienced low hospitalisation rates ranging from 1.1% to 1.8% and an overall mortality rate of 0.01%. This evidence shows that although children were often involved in outbreaks within schools, summer camps and in childcare facilities, severe disease remained uncommon. Reports indicated that while there is an increased risk of severe disease in children with comorbidities, most children who were hospitalised did not report comorbidities. The impact of vaccination in both adults and adolescents has been shown to reduce paediatric hospitalisation rates at a population level, while vaccinated adolescents are less likely to have severe disease than non-vaccinated (36). Whilst post-Delta vaccinations have become more widely accepted, especially more in paediatrics, it would be beneficial to explore this impact on children in the context of hospitalisations, ICU admissions and mortality rates, particularly as newer variants continue to emerge. Given the wide variation in paediatric risk reduction responses within and between nations (19, 21, 27, 64-67) future research is needed into the differential effectiveness of those strategies in curtailing transmission to and amongst children. Finally, the impacts of long-COVID and post-COVID syndrome cannot be discounted and further research should be done to explore the extent of illness and mitigation strategies to prevent incidence of both COVID-19 impacts.
1. World Health Organization. WHO surveillance case definitions for ILI and SARI. January 2014. [Cited 6 October 2022]. Available from: https://www.who.int/teams/global-influenza-programme/surveillance-and-monitoring/case-definitions-for-ili-and-sari.
2. Christopher Troeger BB, Ibrahim A Khalil, Puja C Rao, Jackie Cao, Stephanie R M Zimsen, et al. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis. 2018;18(11):1191-210.
3. World Health Organization. Pneumonia. 2019. [Cited 15 October 2021]. Available from: https://www.who.int/news-room/fact-sheets/detail/pneumonia.
4. Ibrahim LF, Tham D, Chong V, Corden M, Craig S, Buntine P, et al. The characteristics of SARS-CoV-2-positive children who presented to Australian hospitals during 2020: a PREDICT network study. Med J Aust. 2021;215(5):217-21.
5. Molteni E, Sudre CH, Canas LS, Bhopal SS, Hughes RC, Antonelli M, et al. Illness duration and symptom profile in a large cohort of symptomatic UK school-aged children tested for SARS-CoV-2. medRxiv. 2021:2021.05.05.21256649.
6. Mehta NS, Mytton OT, Mullins EWS, Fowler TA, Falconer CL, Murphy OB, et al. SARS-CoV-2 (COVID-19): What Do We Know About Children? A Systematic Review. Clin Infect Dis. 2020;71(9):2469-79.
7. Li B, Zhang S, Zhang R, Chen X, Wang Y, Zhu C. Epidemiological and Clinical Characteristics of COVID-19 in Children: A Systematic Review and Meta-Analysis. Front Pediatr. 2020;8:591132.
8. Cui X, Zhao Z, Zhang T, Guo W, Guo W, Zheng J, et al. A systematic review and meta-analysis of children with coronavirus disease 2019 (COVID-19). J Med Virol. 2021;93(2):1057-69.
9. World Health Organization. Tracking SARS-CoV-2 variants. 2021. [Cited 12 October 2021]. Available from: https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/.
10. World Health Organization. Weekly epidemiological update on COVID-19 - 13 October 2021. 2021. [Cited 13 October 2021]. Available from: https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---13-october-2021.
11. Campbell F, Archer B, Laurenson-Schafer H, Jinnai Y, Konings F, Batra N, et al. Increased transmissibility and global spread of SARS-CoV-2 variants of concern as at June 2021. Euro Surveill. 2021;26(24).
12. Earnest R, Uddin R, Matluk N, Renzette N, Siddle KJ, Loreth C, et al. Comparative transmissibility of SARS-CoV-2 variants Delta and Alpha in New England, USA. medRxiv. 2021:2021.10.06.21264641.
13. Twohig KA, Nyberg T, Zaidi A, Thelwall S, Sinnathamby MA, Aliabadi S, et al. Hospital admission and emergency care attendance risk for SARS-CoV-2 delta (B.1.617.2) compared with alpha (B.1.1.7) variants of concern: a cohort study. Lancet Infect Dis. 2021.
14. Ong SWX, Chiew CJ, Ang LW, Mak TM, Cui L, Toh M, et al. Clinical and virological features of SARS-CoV-2 variants of concern: a retrospective cohort study comparing B.1.1.7 (Alpha), B.1.315 (Beta), and B.1.617.2 (Delta). Clin Infect Dis. 2021.
15. Fisman DN, Tuite AR. Evaluation of the relative virulence of novel SARS-CoV-2 variants: a retrospective cohort study in Ontario, Canada. Canadian Medical Association Journal. 2021;193(42):E1619-E25.
16. Public Health Ontario. COVID-19: Severity of the Delta (B.1.617.2) Variant in Children. 2021. [Cited 17 October 2021]. Available from: https://www.publichealthontario.ca/-/media/documents/ncov/voc/2021/09/covid-19-severity-delta-children.pdf?sc_lang=en.
17. Simon C. Don’t let delta disrupt learning, expert says. The Harvard Gazette. 2021. [Cited 2 October 2021]. Available from: https://news.harvard.edu/gazette/story/2021/09/delta-shouldnt-stop-kids-from-returning-to-class-expert-says/.
18. Lu D WC. The Guardian Australia: Experts say Delta variant spread among Australian children is concerning in absence of Covid vaccine. 2021. [Cited 19 September 2021]. Available from: https://www.theguardian.com/world/2021/aug/19/experts-say-delta-variant-spread-among-australian-children-is-concerning-in-absence-of-covid-vaccine.
19. McLaws ML. COVID-19 in children: time for a new strategy. Med J Aust. 2021;215(5):212-3.
20. Committee on Infectious Diseases. COVID-19 Vaccines in Children and Adolescents. Pediatrics. 2021;148(2).
21. Department of Health & Social Care. Universal vaccination of children and young people aged 12 to 15 years against COVID-19. 2021. [Cited 19 October 2021]. Available from: https://www.gov.uk/government/publications/universal-vaccination-of-children-and-young-people-aged-12-to-15-years-against-covid-19/universal-vaccination-of-children-and-young-people-aged-12-to-15-years-against-covid-19.
22. Gurdasani D, Bhatt S, Costello A, Denaxas S, Flaxman S, Greenhalgh T, et al. Vaccinating adolescents against SARS-CoV-2 in England: a risk–benefit analysis. Journal of the Royal Society of Medicine. 2021;114(11):513-24.
23. UNESCO. Education: From disruption to recovery Paris: United Nations Educational, Scientific and Cultural Organization. 2021. [Cited 17 October 2021]. Available from: https://en.unesco.org/covid19/educationresponse#schoolclosures.
24. The World Health Organisation. All schools in Europe and Central Asia should remain open and be made safer from COVID-19, say WHO and UNICEF. 2021. [Cited April 15, 2022]. Available from: https://www.euro.who.int/en/media-centre/sections/press-releases/2021/all-schools-in-europe-and-central-asia-should-remain-open-and-be-made-safer-from-covid-19,-say-who-and-unicef.
25. American Academy of Pediatrics. AAP continues to advocate measures to allow students to return safely to school. 2021. [Cited April 15, 2022]. Available from: https://publications.aap.org/aapnews/news/6597.
26. Lessler J, Grabowski MK, Grantz KH, Badillo-Goicoechea E, Metcalf CJE, Lupton-Smith C, et al. Household COVID-19 risk and in-person schooling. Science. 2021;372(6546):1092-7.
27. Centers for Disease Control and Prevention. Operational Guidance for K-12 Schools and Early Care and Education Programs to Support Safe In-Person Learning. 2021. [Cited 17 October 2021]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/community/schools-childcare/k-12-childcare-guidance.html.
28. American Academy of Pediatrics. AAP Interim Guidance for Safe Schools (1/5/2021). 2021. [Cited 10 October 2021]. Available from: https://aapca2.org/covid19/.
29. South Africa Department of Health. Disaster Management Act, 2002 Amendments to Regulations Issued in Terms of Section 27(2). 2021. [Cited 15 April 2022]. Available from: https://sacoronavirus.co.za/2022/03/31/disaster-management-act-2002-amendments-to-regulations-issued-in-terms-of-section-272/.
30. Ministry of Health Singapore. FAQS - Masks and personal protective equipment (PPE). 2021. [Cited 10 October 2021]. Available from: https://www.moh.gov.sg/covid-19/general/faqs---masks-and-personal-protective-equipment-(ppe)#:~:text=Children%20of%206%20years%20of,shields%20in%20place%20of%20masks.
31. ABC News. What are the face mask and testing rules for children at school? 2022. [Cited 15 April 2022]. Available from: https://www.abc.net.au/news/2022-01-31/face-mask-testing-rules-kids-school/100792216.
32. United Kingdom Government. Face coverings: when to wear one, exemptions and what makes a good one. 2021. [Cited April 15, 2022]. Available from: https://www.gov.uk/government/publications/face-coverings-when-to-wear-one-and-how-to-make-your-own#full-publication-update-history.
33. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Bmj. 2021;372:n71.
34. Joanna Briggs. Critical Appraisal Tools. 2022. [Accessed 18 October 2022]. Available from: https://jbi.global/critical-appraisal-tools.
35. American Academy of Pediatrics and the Children’s Hospital Association. Children and COVID 19: State Data Report. American Academy of Pediatrics and the Children’s Hospital Association; 2021 09/23/21.
36. National Centre for Immunisation Research and Surveillance. COVID-19 in schools and early childhood education and care services – the experience in NSW: 16 June to 31 July 2021. Sydney: NSW Health; 2021 08/09/21.
37. Nathan N, Prevost B, Lambert S, Schnuriger A, Corvol H. SARS-CoV-2 variant Delta infects all 6 siblings but spares Comirnaty (BNT162b2, BioNTech/Pfizer)-vaccinated parents. J Infect Dis. 2021.
38. Delahoy MJ, Ujamaa D, Whitaker M, O’Halloran A, Anglin O, Burns E, et al. Hospitalizations Associated with COVID-19 Among Children and Adolescents: COVID-NET, 14 States, March 1, 2020–August 14, 2021. Centers for Disease Control and Prevention; 2021.
39. Siegel DA, Reses HE, Cool AJ, Shapiro CN, Hsu J, Boehmer TK, et al. Trends in COVID-19 Cases, Emergency Department Visits, and Hospital Admissions Among Children and Adolescents Aged 0-17 Years - United States, August 2020-August 2021. MMWR Morb Mortal Wkly Rep. 2021;70(36):1249-54.
40. Lam-Hine T, McCurdy SA, Santora L, Duncan L, Corbett-Detig R, Kapusinszky B, et al. Outbreak Associated with SARS-CoV-2 B.1.617.2 (Delta) Variant in an Elementary School - Marin County, California, May-June 2021. MMWR Morb Mortal Wkly Rep. 2021;70(35):1214-9.
41. Dougherty K, Mannell M, Naqvi O, Matson D, Stone J. SARS-CoV-2 B.1.617.2 (Delta) Variant COVID-19 Outbreak Associated with a Gymnastics Facility - Oklahoma, April-May 2021. MMWR Morb Mortal Wkly Rep. 2021;70(28):1004-7.
42. Essa RA, Ahmed SK, Bapir DH, Rasul SA, Khdir AA, Abubakr CP. Clinical features and laboratory findings first case of B. 1.617.2 (delta) variant concern (VOC) in Iraq. Annals of Medicine and Surgery. 2021;69
43. Li H, Lin H, Chen X, Li H, Li H, Lin S, et al. A need of COVID19 vaccination for children aged <12 years: Comparative evidence from the clinical characteristics in patients during a recent Delta surge (B.1.617.2). medRxiv. 2021:2021.11.05.21265712.
44. Fraser S, Ellsworth M, Perez N, Hamilton H, Fletcher S, Brown D, et al. Cerebral Infarctions in an Infant With COVID-19 Delta Variant Infection and Disseminated Tuberculosis. Pediatr Neurol. 2022;126:112-3.
45. Loconsole D, Centrone F, Morcavallo C, Campanella S, Accogli M, Sallustio A, et al. Changing Features of COVID-19: Characteristics of Infections with the SARS-CoV-2 Delta (B.1.617.2) and Alpha (B.1.1.7) Variants in Southern Italy. Vaccines (Basel). 2021;9(11).
46. Cheng QR, Fan MX, Hao J, Hu XC, Ge XH, Hu ZL, et al. Chest CT features of children infected by B.1.617.2 (Delta) variant of COVID-19. World J Pediatr. 2022;18(1):37-42.
47. Tonzel JL, Sokol T. COVID-19 Outbreaks at Youth Summer Camps - Louisiana, June-July 2021. MMWR Morb Mortal Wkly Rep. 2021;70(40):1425-6.
48. Kumar A, Asghar A, Raza K, Narayan RK, Jha RK, Satyam A, et al. Demographic characteristics of SARS-CoV-2 B.1.617.2 (Delta) variant infections in Indian population. medRxiv. 2021:2021.09.23.21263948.
49. Glatman-Freedman A, Hershkovitz Y, Kaufman Z, Dichtiar R, Keinan-Boker L, Bromberg M. Effectiveness of BNT162b2 Vaccine in Adolescents during Outbreak of SARS-CoV-2 Delta Variant Infection, Israel, 2021. Emerg Infect Dis. 2021;27(11):2919-22.
50. Olson SM, Newhams MM, Halasa NB, Price AM, Boom JA, Sahni LC, et al. Effectiveness of Pfizer-BioNTech mRNA Vaccination Against COVID-19 Hospitalization Among Persons Aged 12-18 Years - United States, June-September 2021. MMWR Morb Mortal Wkly Rep. 2021;70(42):1483-8.
51. Molteni E, Sudre CH, Canas LS, Bhopal SS, Hughes RC, Chen L, et al. Illness characteristics of COVID-19 in children infected with the SARS-CoV-2 Delta variant. medRxiv. 2021:2021.10.06.21264467.
52. Naleway AL, Groom HC, Crawford PM, Salas SB, Henninger ML, Donald JL, et al. Incidence of SARS-CoV-2 Infection, Emergency Department Visits, and Hospitalizations Because of COVID-19 Among Persons Aged ≥12 Years, by COVID-19 Vaccination Status - Oregon and Washington, July 4-September 25, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(46):1608-12.
53. Edward PR, Lorenzo-Redondo R, Reyna ME, Simons LM, Hultquist JF, Patel AB, et al. Severity of Illness Caused by Severe Acute Respiratory Syndrome Coronavirus 2 Variants of Concern in Children: A Single-Center Retrospective Cohort Study. medRxiv. 2021.
54. Hao J, Hu X-C, Fan M-X, Chen J, Cheng Q-R, Li Z, et al. Analysis of clinical characteristics of 66 pediatric patients with B.1.617.2 (Delta) variant of COVID-19. World journal of pediatrics : WJP. 2022;18(5):343-9.
55. Bundle N, Dave N, Pharris A, Spiteri G, Deogan C, Suk JE, et al. COVID-19 trends and severity among symptomatic children aged 0–17 years in 10 European Union countries, 3 August 2020 to 3 October 2021. 2021;26(50):2101098.
56. United States Census Bureau. U.S. Adult Population Grew Faster Than Nation’s Total Population From 2010 to 2020. 2022. [Cited 1 May 2022]. Available from: https://www.census.gov/library/stories/2021/08/united-states-adult-population-grew-faster-than-nations-total-population-from-2010-to-2020.html#:~:text=By%20comparison%2C%20the%20younger%20population,from%2074.2%20million%20in%202010.
57. Sixth Tone. China Updates Quarantine Rules, Treatments for COVID-19 Patients. 2022. [Cited 1 May 2022]. Available from: https://www.sixthtone.com/news/1009909/china-updates-quarantine-rules%2C-treatments-for-covid-19-patients%20or%20Dong%202021%20DOI=10.3389/fmed.2021.655231.
58. UNICEF. COVID-19 confirmed cases and deaths New York: UNICEF. 2021. [Cited 16 November 2021]. Available from: https://data.unicef.org/resources/covid-19-confirmed-cases-and-deaths-dashboard/.
59. Hyde Z. COVID-19, children and schools: overlooked and at risk. Medical Journal of Australia. 2020;213(10):444-6.e1.
60. Murdoch Children's Research Institute. COVID-19 and Children’s Surveillance Report. 2021.
61. NSW Health Pathology. NSW respiratory surveillance reports - COVID-19 and influenza. 2021. [Cited 25 April 2022]. Available from: https://www.health.nsw.gov.au/Infectious/covid-19/Pages/weekly-reports.aspx.
62. Gurdasani D, Bhatt S, Costello A, Denaxas S, Flaxman S, Greenhalgh T, et al. Vaccinating adolescents against SARS-CoV-2 in England: a risk–benefit analysis. 2021;114(11):513-24.
63. Allen H, Vusirikala A, Flannagan J, Twohig KA, Zaidi A, Chudasama D, et al. Household transmission of COVID-19 cases associated with SARS-CoV-2 delta variant (B.1.617.2): national case-control study. The Lancet Regional Health – Europe.
64. Cooperative Governance of Traditional Affairs South Africa. Disaster Management Act (57/2002): Amendment of Regulations issued in terms of Section 27 (2). 2021. [Cited 2 November 2021]. Available from: https://www.cogta.gov.za/index.php/2021/06/28/disaster-management-act-57-2002-amendment-of-regulations-issued-in-terms-of-section-27-2-4/.
65. New South Wales Government. Public Health (COVID-19 General) Order 2021. 2021. [Cited 20 December 2021]. Available from: https://legislation.nsw.gov.au/file/Public%20Health%20(COVID-19%20General)%20Order%202021_211110.pdf.
66. Department of Health & Social Care. Face coverings: when to wear one, exemptions, and how to make your own. 2021. [Cited 1 October 2021]. Available from: https://www.gov.uk/government/publications/face-coverings-when-to-wear-one-and-how-to-make-your-own/face-coverings-when-to-wear-one-and-how-to-make-your-own.
67. Public Health England Press Office. JCVI issues updated advice on COVID-19 vaccination of children aged 12 to 15 [press release]. 2021. [Cited 15 October 2021]. Available from: https://www.gov.uk/government/news/jcvi-issues-updated-advice-on-covid-19-vaccination-of-children-aged-12-to-15